
Cobalt Oxide Paura Pango Co3O4 Paura
Whakaahuatanga Hua
Ko te Co3O4 he paura pango, hina-pango ranei.Ko te kiato rahi he 0.5-1.5g/cm3, me te kiato tap he 2.0-3.0g/cm3.Ko te konukawata tetroxide ka taea te rewa i roto i te waikawa pungatara wera, engari kare e rewa i roto i te wai, te waikawa nitric me te waikawa pūhaumāota i te pāmahana rūma.Ka wera ki runga ake i te 1200 ℃, ka pirau ki te waikura cobalt.Ina whakamahana ki te 900°C i roto i te mura o te hauwai, ka heke ki te konukawata cobalt.
Ko te paura konukawata konukawata ko nga ahuatanga o te rahi matūriki iti, te tohatoha rite, te rahi o te waahanga mata motuhake, te teitei o te mahi mata, te iti o te ngoikore, te iti o te poke, te porowhita me te teitei o te mata motuhake, me etahi atu. , a ka taea te whakamahi whanui i roto i nga waahanga hiko, matū me te koranu.
Whakatakotoranga
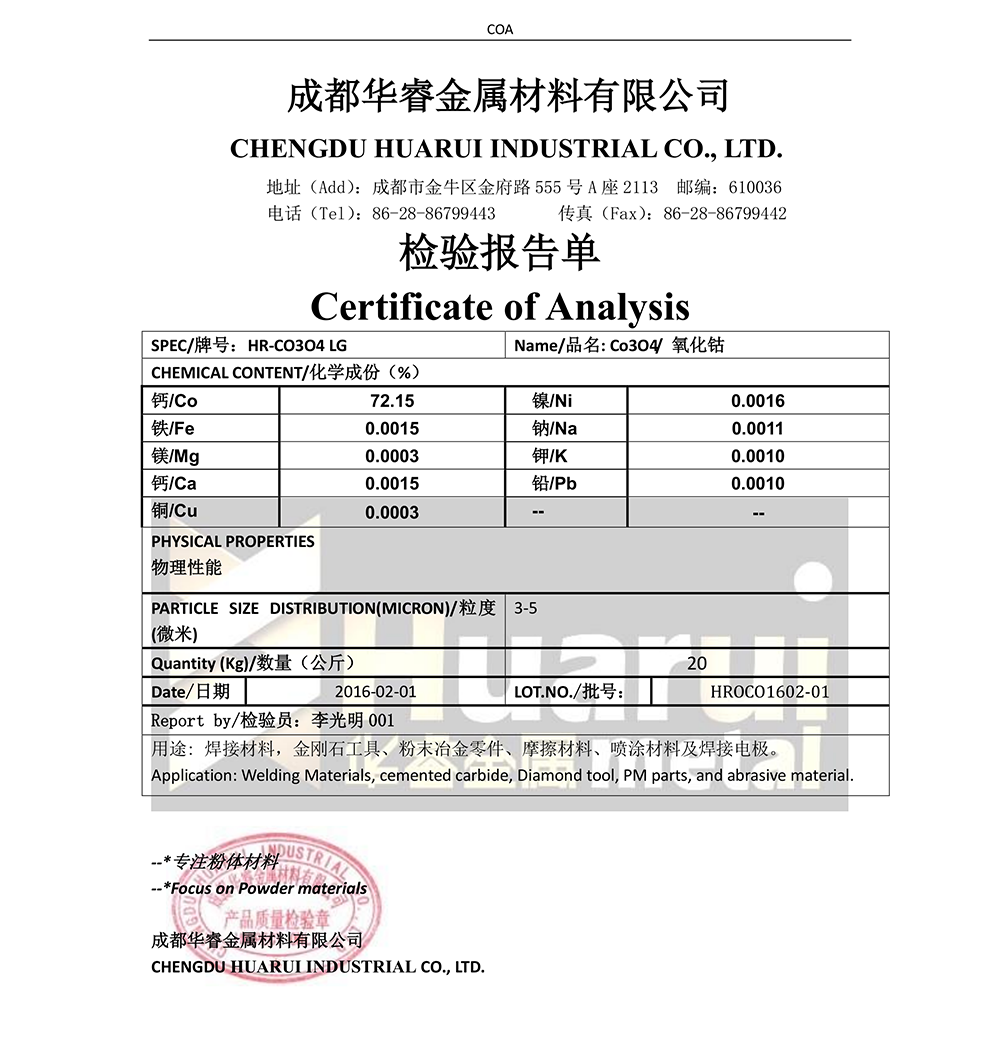
| Te hanganga o te paura konukawata | ||||||
| Kōeke | Ko te poke kei roto (wt% max) | |||||
| Co% | Ni% | Cu% | Mn% | Zn% | Fe% | |
| A | 73.5±0.5 | ≤0.05 | ≤0.003 | ≤0.005 | ≤0.005 | ≤0.01 |
| B | ≥74.0 | ≤0.05 | ≤0.05 | ≤0.05 | ≤0.05 | ≤0.1 |
| C | ≥72.0 | ≤0.15 | ≤0.10 | ≤0.10 | ≤0.10 | ≤0.2 |
COA
Taupānga
1. Ka whakamahia hei karaehe mo te karaehe me nga karaehe, he koranu uaua;
2. Te hāora me te whakakōkī i roto i te ahumahi matū;
3. Ka whakamahia i roto i te ahumahi semiconductor, te hiko hiko, te lithium katote pākahiko cathode rauemi, rauemi autō, pāmahana me te hau pūoko;
4. Ka whakamahia hei reagent tātari parakore teitei, te waikura cobalt me te whakarite tote cobalt
Pūnaha whakahaere kounga

Kei a Huarui te punaha whakahaere kounga.Ka whakamatauhia a maatau hua i te tuatahi i muri i to maatau mahi, ka whakamatau ano i mua i nga tukunga katoa, tae noa ki nga tauira.A, ki te hiahia koe, ka whakaae matou ki te hunga tuatoru hei whakamatautau.Ae, ki te pai koe, ka taea e matou te whakarato tauira ki a koe hei whakamatautau.
Ko te kounga o o maatau hua ka whakamanahia e Sichuan Metallurgical Institute me Guangzhou Institute of Metal Research.Ko te mahi tahi mo te wa roa ki a raatau ka taea te penapena i nga waa whakamatautau mo nga kaihoko.











